Cribriform Pattern Prostate Cancer and Lymphovascular Invasion: Connecting the Dots; a Message of Hope from Pathologists to Men with Prostate Cancer
By Shifaa’ Al Q’aqa’, MD
Prostate cancer is the most common malignancy among men in the United States and Canada. Traditional factors such as cancer extent (stage) and differentiation grade are employed to assess prostate cancer prognosis, but they lack accuracy. In this study recently published in International Journal of Surgical Pathology, we highlight additional risk factors in prostate cancer: the sieve-like shape of the cancer under the microscope (cribriform pattern) and the number of the cancerous foci within the vascular spaces. We hope to increase men’s awareness of prostate cancer and to provide clinicians with valuable measures to consider when treating their patients.
Metastatic spread to other organs is a life-threatening complication of cancer. To metastasize, cancer cells must be able to access blood vessels or lymphatic vessels. Cancer cells will then be carried by the lymph fluid or blood stream and transported to body sites elsewhere, either nearby or distant from the original site. Here, they may proliferate and form cancer metastases. For patients with prostate cancer, the main risk is the development of metastatic disease in bone. If we could accurately predict which patients would most likely develop metastatic disease, this might help clinicians to decide how best to treat patients.
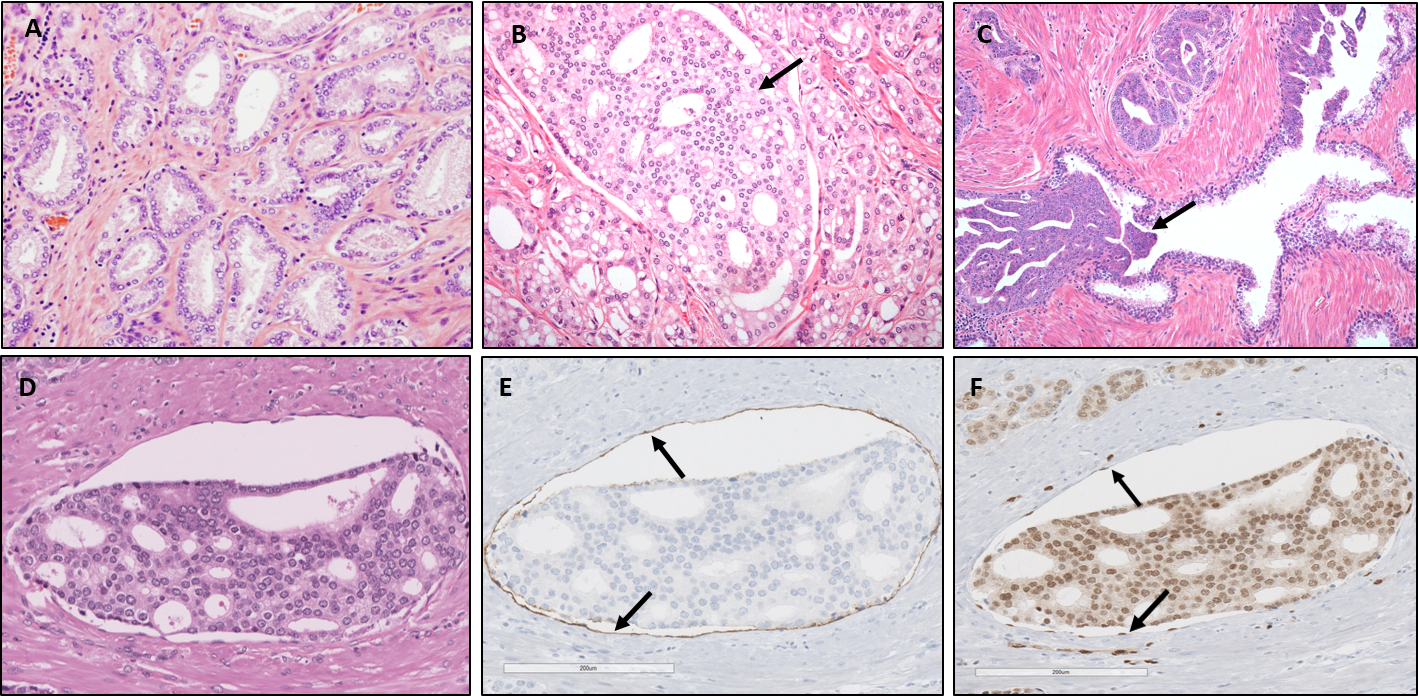
Prostate cancer is unique in that at microscopic level the carcinoma may manifest a large variety of architectures (Figure 1). One recently recognized prostate cancer architecture is the so-called cribriform pattern (https://journals.lww.com/anatomicpathology/Fulltext/2018/01000/ The_New_Realization_About_Cribriform_Prostate.4.aspx). This pattern is characterized by a sheet-like growth of carcinoma cells with multiple glandular openings (lumina) that appear “punched-out”, giving the cancer a sieve-like appearance (Figure 1B). Closely related to this cribriform architecture is intraductal carcinoma, which represents the spread of carcinoma cells within antecedent prostate ducts and glands (Figure 1C). Intraductal carcinoma also commonly has a cribriform appearance. Both the presence of cribriform pattern carcinoma and intraductal carcinoma have recently been established as important predictors of unfavorable prognosis, including metastatic disease. Therefore, their presence is now routinely reported by pathologists when they examine prostate biopsies and surgical prostate specimens.
Figure 1:
A: Prostate cancer displaying simple tubular structures, also known as Gleason grade 3 carcinoma. This architectural pattern, if alone, is associated with indolent prostate cancer. B: The arrow points at a sheet of carcinoma cells with punched out lumina (holes), representing cribriform pattern prostate cancer. This architecture is associated with unfavorable prognosis. C: The arrow points at a carcinoma extending into an antecedent prostate glandular duct, known as intraductal carcinoma. D: A sheet of cribriform pattern carcinoma within a vascular structure. E,F: The vascular lining by endothelial cells is highlighted by a special staining (E: D2-40, F: ERG), indicated by the arrows.
The images were taken at University Health Network, Toronto General Hospital.
Microscopic examination of surgically removed prostates (radical prostatectomy) as a treatment for prostate cancer can reveal presence of cancer cells within vascular spaces (lymphovascular invasion). It has also been well-established that presence of lymphovascular invasion by cancer cells is associated with increased risk of metastatic disease after the surgery. However, its demonstration is cumbersome, subject to considerable interobserver variability and its presence is underreported by pathologists. Only very few studies analyzed the microscopic features of lymphovascular invasion in the prostate. We closely studied in a series of 50 prostatectomy specimens the microscopic features and the location of lymphovascular invasion. By describing the microscopic features of lymphovascular invasion and pointing out the pitfalls, we hope that pathologists will be able to detect and report lymphovascular invasion more reliably. Our main findings were that cancer located in vascular spaces very often showed a “sieve-like” (cribriform) architecture (see figure 1D-F) and they were mostly located in the tissue immediately at the edge and immediately surrounding the carcinoma. This may also help pathologists to focus on those areas when scrutinizing for the presence of lymphovascular invasion. We further noted that more than 90% of the examined carcinomas with lymphovascular invasion also displayed this cribriform architecture. This might suggest that carcinoma with this cribriform structure is more prone to invade vascular spaces.
Another striking finding of our study was that some prostate cancers displayed very extensive lymphovascular invasion involving up to a maximum of over 100 vascular spaces with cancer emboli. Importantly, a larger number of vascular spaces with cancer was the only factor strongly associated with the occurrence of bone metastases at follow-up of the patient after his surgery. If confirmed in larger studies, this would be a highly clinically significant finding: men with extensive lymphovascular invasion should be offered more intense and precise therapy in the hope to delay their metastatic disease. As a consequence, quantification of lymphovascular invasion during microscopic examination of prostatectomy specimens might be warranted, particularly among those with cribriform pattern prostate cancer.
About the Author